Dou you know environmental hazards of waste lithium-Ion batteries?Waste lithium-ion batteries (LIBs) pose significant environmental risks due to their tendency to undergo various chemical reactions that can lead to pollution. Spent LIBs primarily consist of cathode and anode materials, electrolytes, separators, and binders. If discarded haphazardly, these components can inflict considerable harm on the environment.
Electrolytes, such as lithium hexafluorophosphate (LiPF6), are commonly dissolved in organic solvents like ethylene carbonate (EC), dimethyl carbonate (DMC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC). When a battery is damaged, LiPF6 can react with water to form hydrogen fluoride (HF), according to the following chemical equation:
LiPF6+ H2O → LiF + POF3 + 2HF
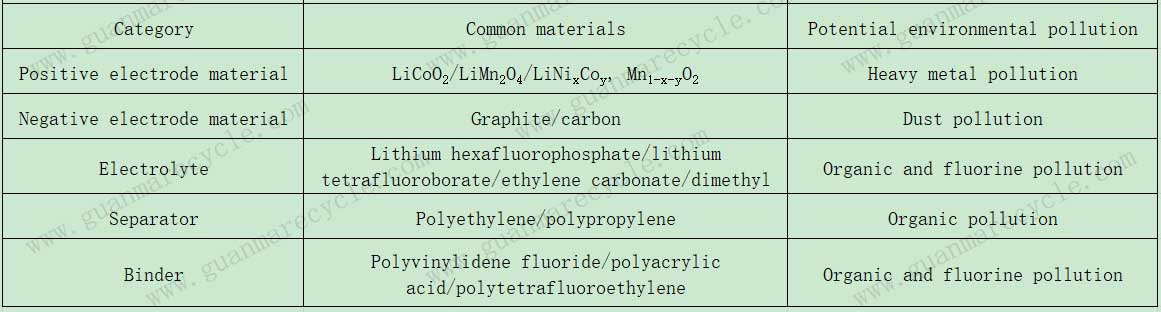
These organic solvents are highly toxic and, upon contact with air or moisture, can volatilize, leading to atmospheric pollution that severely impacts human health. Binders, such as polyvinylidene fluoride (PVDF), and separators made of polypropylene (PP), polyethylene (PE), and vinylidene fluoride (VDF), contribute to organic contamination. Carbon materials and graphite can result in particulate matter pollution, while heavy metals like nickel (Ni), cobalt (Co), and manganese (Mn) found in cathode materials can lead to heavy metal pollution. As such, spent LIBs have become a major source of environmental contamination.
Moreover, the distribution density of spent LIBs correlates positively with population density and economic development. The environmental risks posed by spent LIBs have a substantial impact on public health and economic development. In summary, the recycling of spent LIBs offers multiple benefits in terms of resource recovery, environmental preservation, and social well-being. Amidst the tightening supply of minerals such as cobalt and lithium, devising scientific and efficient methods for recycling valuable metals from spent LIBs has become a crucial subject of study.



